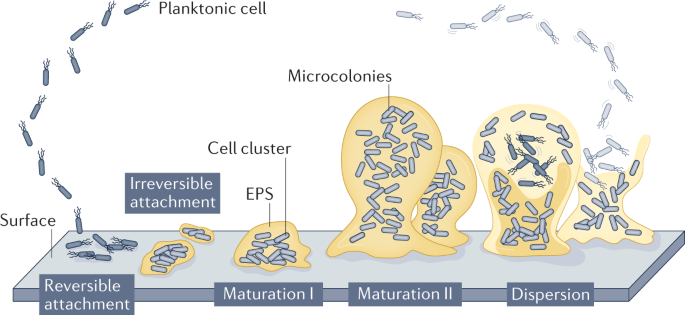
Biofilms are complex microbial communities that adhere to surfaces. They consist of a consortium of microorganisms, including bacteria, fungi and viruses embedded in a self-produced extracellular matrix.
In hospital settings, biofilms can form on various equipment surfaces, posing a risk for healthcare-associated infections (HAIs).
Formation and characteristics of biofilms
Biofilm formation begins with the attachment of microorganisms to a surface. This can occur on hospital equipment such as catheters, ventilators, urinary catheters, and surgical instruments.
Abuja newspapers distributors get new excos
Benue majority leader denies sponsoring ‘fake news’ against SGF
Once attached, microbial colonies start producing an extracellular matrix, which protects them from external stresses and host immune responses. The matrix also helps facilitate the exchange of nutrients, genetic material, and signalling molecules among community members.
How biofilms survive in hospital equipment
Resilience: Biofilms are highly resilient and can tolerate a range of harsh conditions that would be lethal to individual planktonic microorganisms. They can withstand mechanical stress, desiccation, and exposure to antibiotics and disinfectants.
Adherence and colonization: Hospital equipment provides favorable surfaces for biofilm development due to their complex structures, including crevices, rough surfaces, and hard-to-reach areas. Biofilm colonization can occur rapidly, providing a continuous source of pathogens that can cause HAIs.
Antimicrobial resistance: Biofilms display increased resistance to antimicrobial agents, such as antibiotics and disinfectants. The extracellular matrix acts as a physical barrier, preventing these agents from reaching the microbial cells within the biofilm. Additionally, the presence of persister cells, dormant bacteria with high tolerance to antibiotics, further contributes to resistance.
Cross-contamination: Biofilms can serve as reservoirs for pathogens, leading to cross-contamination among patients and hospital surfaces. If not properly managed and cleaned, contaminated hospital equipment can result in the spread of infectious agents.
Challenges in biofilm management
Detection: Biofilms are often difficult to detect visually, as they are invisible to the naked eye. They require specialized techniques, such as microscopy, genetic approaches, or biochemical assays, to identify and quantify their presence on hospital equipment.
Cleaning and disinfection: Traditional cleaning and disinfection methods may not effectively eradicate biofilms. The complex nature of the biofilm matrix and its ability to protect microorganisms hinder the complete elimination of pathogens. Specialized protocols and detergents are needed to effectively remove and inactivate biofilms.
Equipment design: The design of hospital equipment can play a role in biofilm prevention. Smooth, easily cleanable surfaces, as well as removable components, can help reduce the risk of biofilm formation and improve cleaning effectiveness.
Challenge to preventing healthcare-associated infections
The survival of biofilms in hospital equipment poses a significant challenge in preventing healthcare-associated infections.
Biofilms are highly resilient, display antimicrobial resistance, and can serve as sources of cross-contamination.
Proper detection methods, improved cleaning protocols, and equipment design considerations are essential for effective biofilm management in healthcare settings.
Continued research and advancements in this field are crucial for enhancing patient safety and reducing the transmission of infections within hospitals.
Biofilms on hospital equipment pose several associated risks, including:
Infection control: Biofilms can act as reservoirs for pathogenic microorganisms, leading to an increased risk of healthcare-associated infections (HAIs). These HAIs can be difficult to treat as biofilms offer protection to bacteria, making them resilient to traditional disinfection and antimicrobial methods.
Device damage: Biofilms can damage hospital equipment over time. They can cause corrosion, blockages, and impair the functionality of various medical devices, resulting in equipment malfunction or failure. This can compromise patient care and increase healthcare costs.
Reduced efficiency: Biofilms can interfere with the normal functioning of hospital equipment, leading to reduced efficiency and accuracy of diagnostic and therapeutic procedures. This can delay patient care and impact treatment outcomes.
Contamination spread: Biofilms can act as sources of microbial contamination, enabling the spread of pathogens within healthcare settings. If biofilms are not properly addressed, they may contribute to cross-contamination between patients, healthcare workers, and various hospital surfaces.
Antibiotic resistance: Biofilms possess increased resistance to antibiotics, as the structure of the biofilm protects bacteria from the effects of antimicrobial agents. This can contribute to the development of antibiotic-resistant strains, making infections more difficult to treat and control.
How to eradicate biofilms on hospital equipment
To mitigate these risks, healthcare facilities need to implement effective cleaning and disinfection protocols, consider the use of antimicrobial materials for equipment, promote regular maintenance, and utilize advanced technologies designed to prevent and control biofilm formation.
To eradicate biofilms on hospital equipment, it’s important to follow a thorough cleaning and disinfection process. Here are some steps to consider:
Pre-cleaning: Remove any visible organic matter from the equipment using disposable towels, brushes, or sponges. This step helps ensure that the disinfectant can reach the biofilm effectively.
Select an appropriate disinfectant: Choose a disinfectant specifically designed to eliminate biofilms. Look for one that is approved for use in healthcare settings and has proven efficacy against the types of biofilms commonly found in hospitals.
Follow product instructions: Read and follow the instructions provided by the manufacturer of the disinfectant. This includes diluting it properly if required and ensuring adequate contact time for maximum effectiveness.
Disinfect the equipment: Apply the disinfectant to all surfaces of the equipment, paying extra attention to areas prone to biofilm formation, such as crevices, joints, and textured surfaces. Ensure that the disinfectant reaches all parts of the equipment.
Allow sufficient contact time: The disinfectant needs enough time to work effectively against biofilms. This duration is typically mentioned in the product instructions. Ensure that the equipment remains wet with the disinfectant for the recommended period.
Rinse and dry thoroughly: After the appropriate contact time, rinse the equipment with clean water to remove any residual disinfectant. Finally, dry the equipment completely before putting it back into service.
Regular maintenance: To prevent biofilm formation in the future, establish a regular cleaning and maintenance schedule for the equipment. This includes periodic deep cleaning, disinfection, and routine checks for any potential biofilm growth.
Remember, it’s crucial to consult with your facility’s infection control guidelines and follow the protocols established by your healthcare organization for cleaning and disinfection practices.
Please note that the specific procedures and disinfectants used may vary depending on the equipment and the hospital’s policies.
Gurama, is a Fellow Doctor writing from Faculty of Pharmaceutical Sciences, Gombe State University