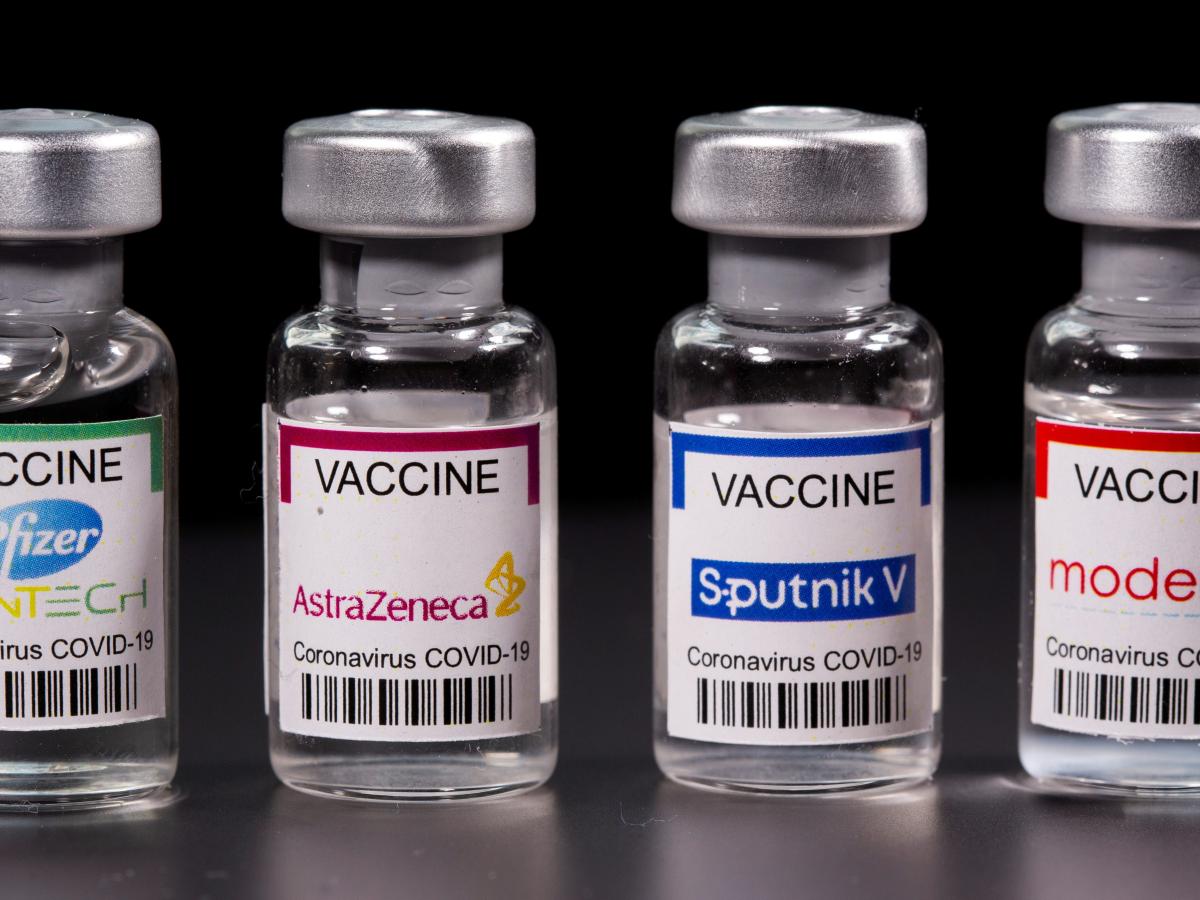
On March 2, Nigeria became the third West African country, after Ghana and Ivory Coast, to receive nearly four million COVID-19 dosages of the Oxford/AstraZeneca vaccine through the international COVID-19 Vaccines Global Assess Facility (COVAX) scheme. So far, the Nigerian government says it has given out 3.97 million dosages of the AstraZeneca vaccine with 1.42 million people fully vaccinated since it kicked off its nationwide inoculation programme.
Recently, the government also received another four million dosages of the Moderna Covid-19 vaccine donated by the United States of America to help the country battle a third wave of infections. The doses, which arrived on two planes, were received by UNICEF officials on behalf of Nigeria at the Nnamdi Azikiwe International Airport, Abuja. This was followed by another 177,600 Janssen (Johnson & Johnson) vaccine through the African Union on August 12, 2021.
The National Primary Health Care Development Agency (NPHCDA) has said the shipment of the J&J vaccine is the first batch from the African Union which would be received in monthly segments until a total of 29.8 million doses are completed.
What are the 3 brands of vaccines?
Oxford-Astrazeneca
The Oxford-Astrazeneca vaccine is a viral vector vaccine developed by the Oxford University and AstraZeneca – a pharmaceutical company, and given by intramuscular injection. According to Medical News Today, the viral vector vaccine contains the gene that encodes for the spike protein on the surface of the SARS-CoV-2 virus and once delivered to the cells, the gene is transcribed, prompting our cells to make the spike protein. It is the presence of the protein that triggers the body’s immune system to produce antibodies to fight against the spike protein, which prepares the body to fight against Covid-19 should it enter the body. The vaccine has a shelf life of six months stored in a refrigerator between 2 to 8°C and once removed from fridge, may be stored between 2 to 25°C for up to six hours.
Moderna Covid-19 vaccine
The Moderna COVID‑19 vaccine is codenamed mRNA-1273 and developed by Moderna, the United States National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA). The Moderna is an mRNA vaccine that sends the body’s cells instructions for making a spike protein that will train the immune system to recognize it. The immune system will then attack the spike protein the next time it sees one. According to the company, the vaccine has greater than 90 per cent efficacy against cases of COVID-19 and more than 95 per cent against severe cases, with approximately six months median follow-up after the second dose.
Johnson and Johnson
Johnson and Johnson vaccine is the third vaccine to be granted emergency use authorization by the US Food and Drugs Administration after Pfizer and Moderna. Unlike the Pfizer and Moderna however, the Johnson and Johnson, like Oxford-Astrazeneca are viral vector vaccines which use a modified, harmless version of a different virus as a vector, or carrier, to deliver immunity instructions to cells in the body. The body then follows those instructions to build an immune response to the intended virus (in this case SARS-CoV-2, which causes COVID-19.)
Between Oxford-AstraZeneca, Moderna and Johnson & Johnson
There are few differences between the Moderna, J & J and AstraZeneca which are the three vaccines that have been deployed to Nigeria. All three have been granted emergency use authorization by the World Health Organisation (WHO) as well as approved for use in Nigeria by the National Agency for Food and Drug Administration and Control (NAFDAC).
Oxford-AstraZeneca was the first vaccine to be administered in Nigeria and according to a report by the BBC, it is made from a weakened version of a common cold virus (known as an adenovirus) from chimpanzees and has been modified to contain genetic material shared by the coronavirus – although it can’t cause the illness.
The Moderna vaccine which the Nigerian government has begun administering for the second phase of the vaccination programme can be thawed in the refrigerator between 2°C and 8°C (36°F and 46°F) with unpunctured vials stored in the refrigerator for up to 30 days, according to the Centre for Disease Control. The vaccine is also designed to be administered as two 0.5 mL doses given by intramuscular injection with the WHO recommending an interval of 28 days between doses. WHO states that the vaccine has an efficacy of approximately 94.1 per cent in protecting against COVID-19, starting 14 days after the first dose.
The Johnson & Johnson vaccine requires storage at 35.6 to 46.4 degrees F. According to the company, the vaccine is estimated to remain stable for two years at -4°F (-20°C) and for a maximum of three months at routine refrigeration at temperatures of 36-46°F (2 to 8°C).
Speaking on the vaccine, the Executive Director, National Primary Health Care Development Agency, Dr Faisal Shuaib, explained that the temperature for storing the J&J vaccine is that which most of Nigeria’s routine immunization vaccines are stored. A major advantage the Johnson and Johnson vaccine has over Moderna and Oxford-Astrazeneca is that it requires only a single shot which makes it easier to distribute and administer in hard-to-reach areas.
Dr. Shuaib said the first batch of the J&J vaccine will be focused on those who are in the hard-to-reach areas as well as the elderly and frail individuals across the country since it is administered only in one dose.
What Nigerian experts are saying?
As concerns mount over the different brands of Covid-19 vaccines imported into the country, Nigerian experts have allayed fears over the three brands, saying all three have been approved for use by the National Agency for Food and Drug Administration and Control (NAFDAC) and also granted emergency use authorisation by the World Health Organisation (WHO).
The National Primary Health Care Development Agency and the Nigeria Medical Association (NMA) have stressed that the Moderna vaccine, Johnson and Johnson and the Oxford-Astrazeneca have all been approved and are safe and effective against Covid-19.
The Executive Director/CEO of NPHCDA, Dr. Faisal Shuaib, while briefing newsmen in Abuja recently, assured Nigerians that COVID-19 vaccines, regardless of brand, as long as they have been approved by NAFDAC, provide adequate protection against the disease.
The NPHCDA, however, said all those who have taken the Oxford-AstraZeneca vaccine and are due for their second dose would be given their second dose within the month of August when the over 5,000 doses of AstraZeneca will arrive in the country. In other words, Dr. Shuaib stressed that it is not the recommendation of WHO for anyone who takes the first jab of Oxford-AstraZeneca to take the Moderna vaccine which has just been delivered to Nigeria.
Also, the Nigeria Medical Association (NMA) recently allayed fears over the Moderna vaccine, saying it is safe and effective against the COVID-19 virus. The NMA Lagos Chairman on COVID-19, Dr Japhet Olugbogi said: “Moderna has up to 94 per cent ability to prevent people from coming down with symptoms of COVID-19 and also 90 per cent ability of preventing people from coming down with a severe form of the disease.”
 Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.
Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.