| L |
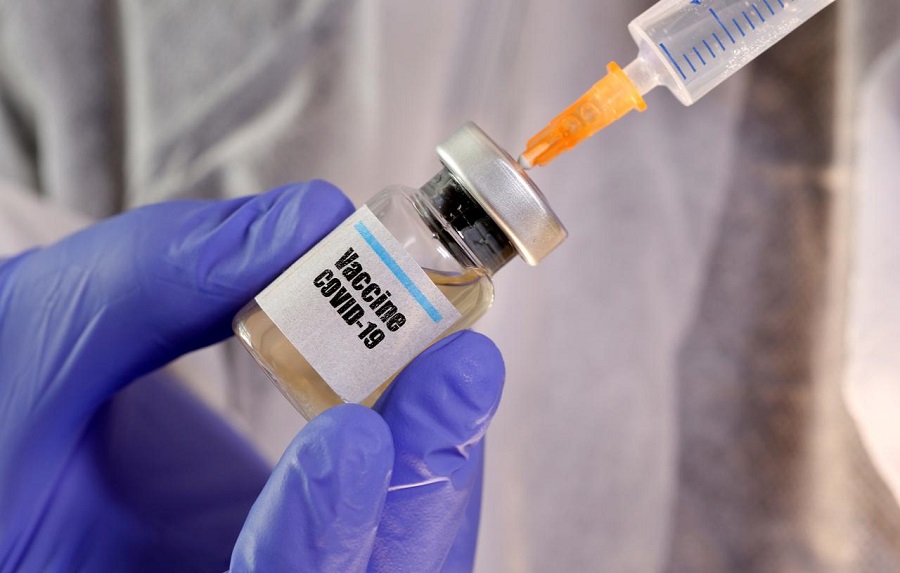
ast week Tuesday, Russian President Vladimir Putin, announced Russia’s approval of the world’s first coronavirus (COVID-19) vaccine, nicknamed Sputnik-V, following less than two months of human testing.
Putin, allaying fears of its authenticity, claimed that one of his adult daughters had been inoculated with the vaccine. Although Russian authorities have offered no proof to back up claims of safety or effectiveness, Putting said: “I know it has proven efficient and forms a stable immunity. We must be grateful to those who made that first step very important for our country and the entire world.”
While Russian officials have said large-scale production of the vaccine wasn’t scheduled until September, Deputy Prime Minister Tatyana Golikova said vaccination of doctors could start as early as this August. Officials say they will be closely monitored after the injections. Mass vaccination may begin as early as October.
“We expect tens of thousands of volunteers to be vaccinated within the next months”, Kirill Dmitriev, chief executive of the Russian Direct Investment Fund that bankrolled the vaccine, told reporters.
The vaccine, developed by the Gamaleya Institute in Moscow with assistance from Russia’s Defense Ministry, uses a different virus — the common cold-causing adenovirus — that’s been modified to carry genes for the “spike” protein that coats the coronavirus, as a way to prime the body to recognise if a real COVID-19 infection comes along.
However, scientists in Russia and other countries sounded an alarm, with Russia’s Association of Clinical Trials Organizations, while urging government officials to postpone approving the vaccine without completed advanced trials, saying that “fast-tracked approval will not make Russia the leader in the race; it will just expose consumers of the vaccine to unnecessary danger.”
Professor Francois Balloux, director of University College London’s Genetics Institute, said the decision to approve the vaccine so quickly was “reckless and foolish. Mass vaccination with an improperly tested vaccine is unethical. Any problem with the Russian vaccination campaign would be disastrous both through its negative effects on health, and also because it would further set back the acceptance of vaccines in the population.”
In contrast to Russia, vaccines entering final-stage testing in US require studies of 30,000 people each. Two vaccine candidates already have begun those huge studies, with three more set to get by fall.
That’s a similar technology as vaccines being developed by China’s CanSino Biologics and Britain’s Oxford University and AstraZeneca.
Amidst the claims and reservations of the new Russian COVID-19 vaccine, the World Health Organisation (WHO) said it had set up discussions with Russian health authorities to work on a process for possible prequalification for the country’s vaccine.
WHO spokesman, Tarik Jasarevic, said: “We are in close contact with Russian health authorities and discussions are ongoing with respect to possible WHO prequalification of the vaccine, but again prequalification of any vaccine includes the rigorous review and assessment of all required safety and efficacy data.”
The announcement of the vaccine is a welcome one as it gives hope that a cure or prevention is in the offing for the pandemic which has completely changed the world as it once was. But the Russian authorities, who are the only ones that know details of the vaccine, must ensure that speed does not compromise the safety of the vaccine.
To stem the reign of skepticisms, transparency must rule the process and vaccine data should be shared openly to enable general assessment of its efficacy and safety. In addition, the vaccine, like all the other ones to come, must go through “robust clinical trials” including Phase III trials to ensure safety and test for any potential rare side effects.
This way, the world would together trust the vaccine and end this COVID-19 pandemic through safe and effective vaccines, treatments and diagnostics for the virus that could be available for all people of the world. Any attempt to use the vaccine for global politics would simply backfire.
 Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.
Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.