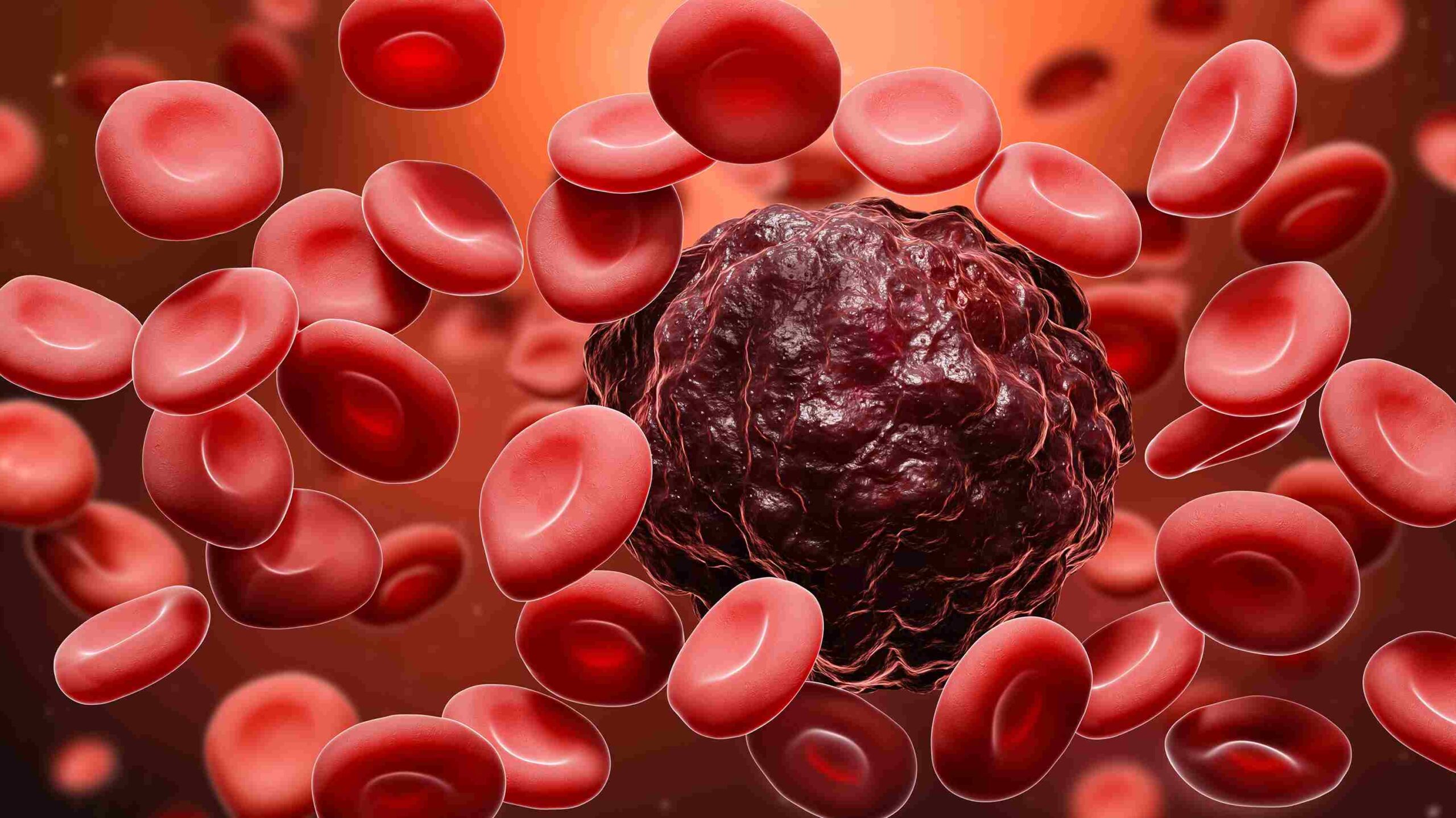
An experimental therapy could offer blood cancer patients a new lease of life, scientists have said.
The immunotherapy treatment, called talquetamab, successfully treated 73 per cent of patients with multiple myeloma in a global trial.
It helped people who had relapsed in their disease more than once after other treatments failed to bring them into remission for an extended period of time.
Dr Ajai Chari of the Multiple Myeloma Programme at the Tisch Cancer Institute and lead author of both studies said, “This means that almost three-quarter of these patients are looking at a new lease on life.”
Bloodbath in S/East: Police mobilize against IPOB terror
Bloodbath in S/East: Police mobilize against IPOB terror
The drug works by teaching the body’s white blood cells to kill tumour cells that form in a type of white blood cell called plasma cell.
Multiple myeloma is a deadly cancer which kills around half of sufferers within five years of diagnosis.
The new drug specifically targets the protein, GPRC5D. Too much of it in the bone marrow is associated with poor survival in patients with multiple myeloma.
Talquetamab binds to GPRC5D which rallies T cells – white blood cells that develop from stem cells in the bone marrow – to fight the cancerous cells.
It was tested in two separate trials, the first of which was published in September.
The phase two results were presented on Saturday at the American Society of Hematology’s annual meeting.
The reflected data from nearly 300 cancer patients who had tried at least three different therapies without achieving lasting remission.
While 143 patients were treated with a 405 μg per kilogramme dose weekly and 145 patients treated with a higher 800 μg per kilogramme dose every other week.
Scientists recorded improvements in symptoms after about a month. People began responding to the treatments at different doses within about nine months.
More than 30 per cent of patients in both groups had a complete response, meaning all signs of cancer have disappeared.
The rest of successful treatment patients had a very good partial response or better, meaning the cancer was substantially reduced but not necessarily down to zero.
Talquetamab also worked at least as well or better than other standard treatments for multiple myeloma.
Sixty-five per cent of those who received talquetamab at the 405-μg dose level and 70 per cent of those who received it at the 800-μg dose level had an immune response.
Meanwhile, only 25 per cent of similar patients have received selinexor plus dexamethasone, and 31 per cent of those who received belantamab mafodotin.
Both of those drugs are Food and Drug Administration-approved.
Mail Online
 Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.
Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.


