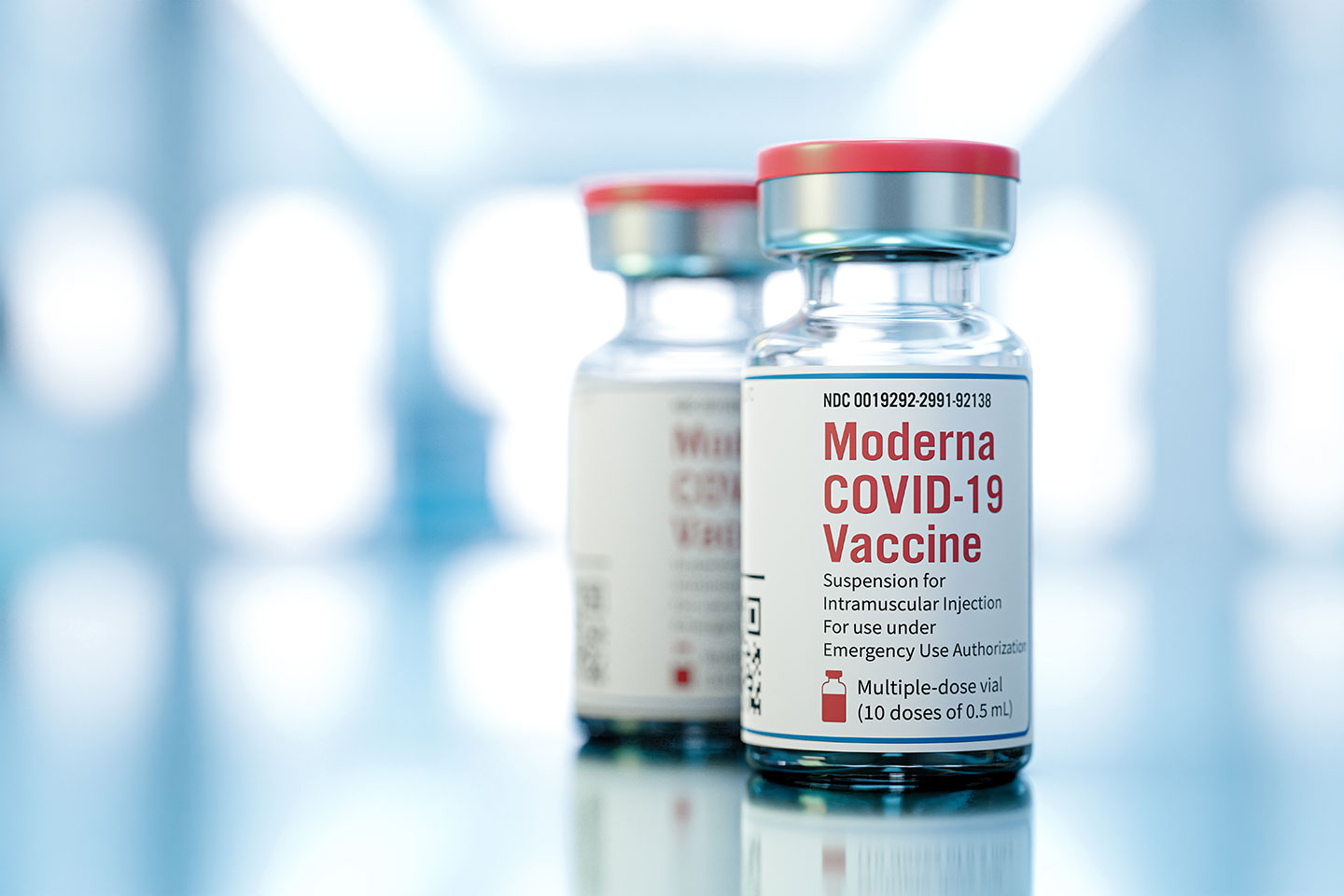
The National Agency for Food and Drug Administration and Control (NAFDAC) has granted emergency use authorization for Moderna and AstraZeneca COVID-19 vaccines in the country.
It however granted conditional approval for Sputnik V COVID-19 vaccine.
The Director-General of the agency, Prof Moji Adeyeye, told a briefing in Abuja, “NAFDAC is announcing the approval of Moderna and AstraZeneca vaccines and conditional approval of Sputnik V vaccine.”
Adeyeye said the NAFDAC Vaccine Committee has been carefully assessing several the vaccines despite the fact the vaccines have been approved by stringent regulatory countries or have received WHO’s Emergency Use Listing (EUL).
She said a COVID-19 vaccine that has gone through the prior approval from either of these two sources had gone through quality, safety and efficacy evaluation which is a prerequisite for acceptance by COVAX Facility.
She added that most regulatory agencies across the world use this mechanism to expedite their own regulatory approval to import and administer COVID-19 vaccines.
According to her, most Moderna and AstraZeneca AZD1222 vaccines have received WHO EUL listing and were given expedited approvals.
She said Sputnik V is yet to receive the EUL approval and therefore was subjected to full six-months review by NAFDAC.
The agency was granted access to the dossiers and prior assessment reports of Moderna and AstraZeneca from the WHO website at different times over the past two months, she said.
She said NAFDAC is the first national regulatory agency in Africa to have Guidance on Regulatory Preparedness for Emergency Use Authorization (EUA) licensing or access to COVID-19 vaccines, adding that it also gives full reviews for vaccines that have not gone through emergency use Listing route.
 Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.
Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.


