Dr Nnakelu Eriobu is the Principal Investigator of In-Patient Treatment with Anti-Corona Virus Immunoglobulin (ITAC) Study, a research on COVID-19 treatment being conducted by the Institute of Human Virology, (IHVN) Nigeria. In this interview, he explains why Nigeria is the only African country in the study; the significance to COVID-19 treatment, and what the government can do to address COVID-19 vaccine hesitancy, among others.
What is the ITAC study about?
- Women’s March: Celebrating Oby’s passion for a new Nigeria
- Okorocha’s Son-In-Law Shot During Brawl Over Seized Property To Be Flown Abroad
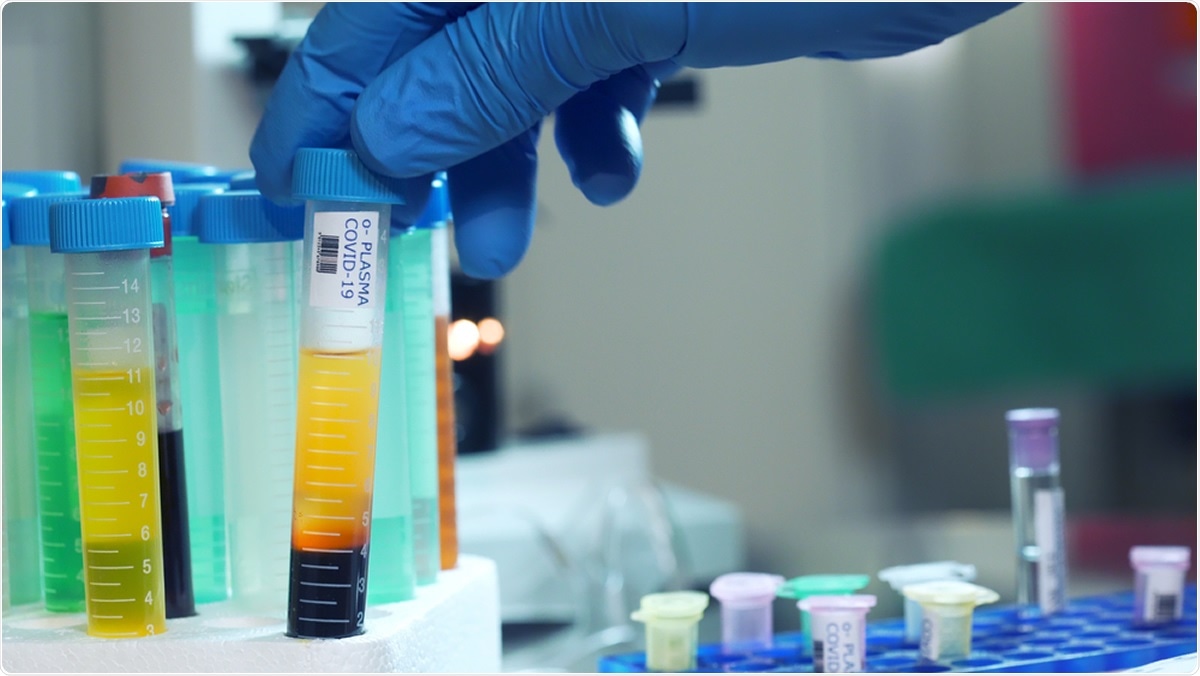
The ITAC study is an international multi-sized clinical trial that is assessing the safety of the use of immunoglobulin treatment on hospitalised COVID-19 patients.
It is assessing the efficacy and safety of intravenous immunoglobulin treatment in hospitalised COVID-19 patients with mild to moderate symptoms.
Intravenous immunoglobulins are antibodies that are harvested from plasma of patients who have recovered from COVID-19.
It is harvested from their blood and undergoes some pharmaceutical processes where it is made safe for other human use.
The viruses, bacteria, parasites and infectious agents are removed including HIV, Hepatitis B,C.
It undergoes some pharmaceutical stabilization processes and they are packaged into vials. This protein contains active antibodies that can fight the COVID 19 viruses when given to another patient who is currently fighting COVID-19 and has symptoms.
The study started globally in November 2020 and is happening in about 18 countries and we have about 60 sites that are part of this study in these countries.
Some countries have two or more sites. IHVN is actually the only site in Nigeria and Nigeria is the only site in Africa, so we are really privileged to be part of this study. So Nigeria is actually the only African country that is on the ITAC study.
Aside Nigeria, the other countries involved in the study are in Europe. They are Argentina, Belgium, Brazil, Denmark, France, Germany, Greece, Israel, Japan, Mexico, Peru, Poland, Portugal, Spain, Thailand, United States and the United Kingdom.
What is the significance of this study?
We are in the middle of a pandemic, a serious one at that, with thousands of lives being lost daily across the globe and there is no treatment for COVID-19 yet.
The scientific community has tried a couple of antiviral agents that they felt might have some effects against COVID-19 but they have not been effective.
So there is an urgent and unmet need for a verifiable, efficacious treatment to counter COVID-19 infection in people who are symptomatic. That is why we are studying this drug, to see if it is safe and efficacious.
Already pre-existing scientific knowledge has shown that when you collect anti-bodies from patients who have recovered and infuse those antibodies in a patient who is currently having that same infection, it has an action against the viruses that cause the infection and can improve the recovery process.
So that is exactly what we are doing here. If this approach is seen to work, then it is going to be scaled up as treatment across many countries.
In the US currently, they are already putting this treatment to work and it seems like people are improving but we need more data.
This study is going to provide more robust data to say that the intravenous immunoglobulin has some action against COVID-19 infection in symptomatic patients, and could actually be one of the treatment approaches to this current pandemic.
Why is Nigeria considered among the 18 countries for the study?
The reason Nigeria is considered is because of the Institute of Human Virology (IHVN). Over the years, IHVN has been able to demonstrate its competence in clinical research. This would not be the first international multisite research it has done.
IHVN was part of the Strategic Timing of Antiretroviral Treatment (START) study, that had over 20 countries and 113 sites and we were able to demonstrate capacity and competence in providing good clinical outcomes in terms of our data, good clinical practice, the quality of implementation and all that.
We have done well in the past and IHVN is currently also part of another study called the D2EFT (Doultegravir and Darunavir Evaluation in Adults Failing Therapy).
Overtime, as we build international collaboration, IHVN has been able to distinguish itself as a centre of excellence and once you have a good track record, it is very easy to continue to attract funding. I think that is the reason these people feel comfortable with allowing IHVN to be a part of this international high level study.
With the rate of conspiracy theories and hesitancy with COVID-19 vaccines, what is your advice for the people and the government?
The conspiracy theories are giving people a lot of wrong advice.
The vaccines are safe and they have saved and improved lives. With vaccines, we see children dying less than they used to.
So if you are at risk of getting COVID-19 because you are a health worker, because you are in the older age group or because you are in a community with high prevalence, then you need to get the vaccine.
I think policy makers and government officials should bring themselves forward and take these vaccines publicly. It would be beneficial if officials like the president, and minister of health take the vaccine in public view.
We saw how difficult it was for some parts of Nigeria to adopt the polio vaccine but now that is gradually being overcome.
With time, transparency, accountability and gradual persistence in preaching the right message to the public, people will get to a point where they will be comfortable to get the vaccines.
 Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.
Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.