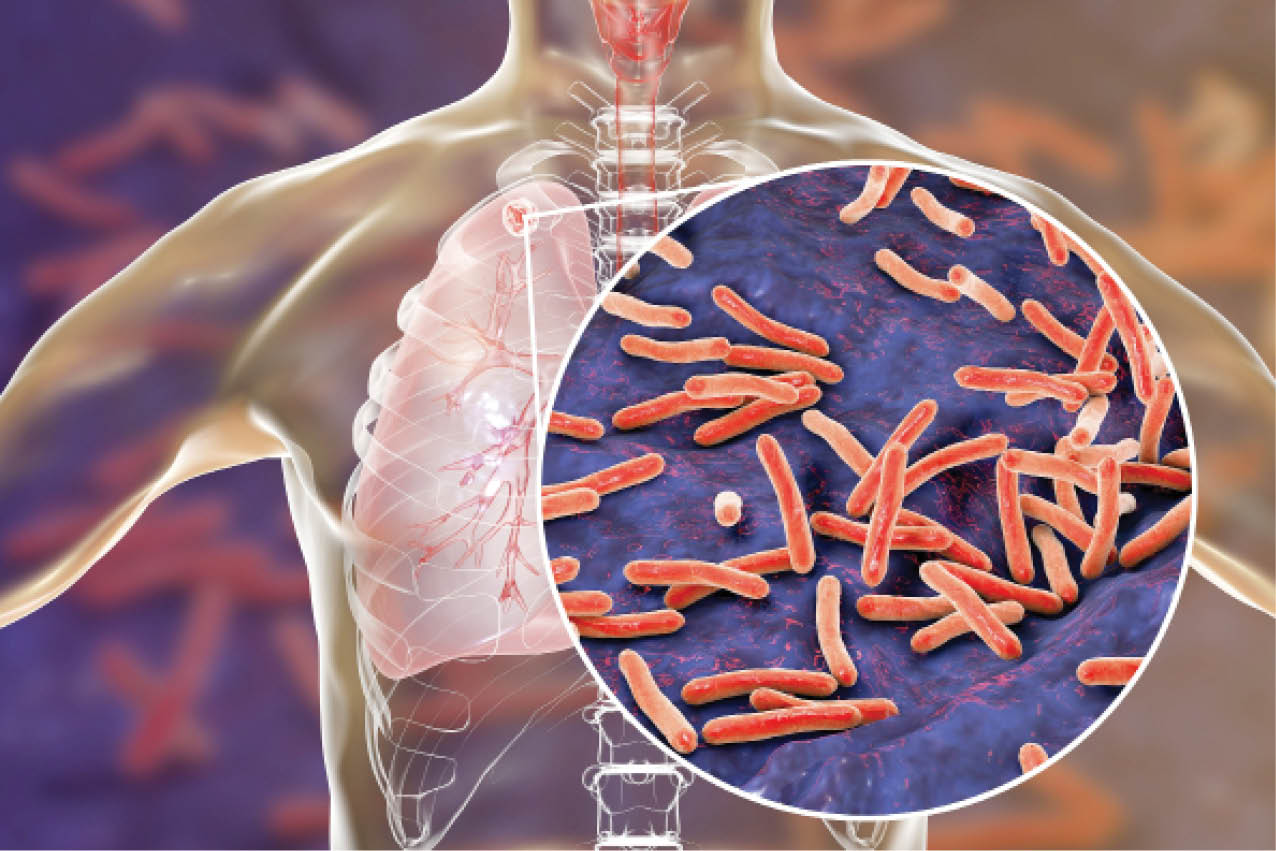
Think of humanity’s worst plagues, and the Black Death, the Spanish flu, and COVID-19 all come to mind. Millions have died in those deadly pandemics, but their toll pales in comparison with that of tuberculosis (TB), which has killed more than 1 billion people over the past 2000 years—and still kills 1.5 million people worldwide every year. But how and when TB got to be so deadly has long been a mystery.
Now, by tracing the evolution of a gene variant that makes people more susceptible to the disease, researchers have been able to track the rise and fall of TB over the past 10,000 years—and have shown how it reshaped the immune systems of people living in Iron Age Europe. “We are [all] the descendants of people who survived past epidemics,” says author Lluis Quintana-Murci, a population geneticist at the Pasteur Institute and the College of France. This paper helps identify “which are the true pathogens that have changed our DNA and made us more resilient.”
The earliest evidence of TB comes from skeletons buried in the Middle East 9000 years ago, soon after the invention of agriculture. But the variant that kills humans today—Mycobacterium tuberculosis—emerged 2000 years ago, when people lived in denser settlements alongside domesticated animals, often reservoirs for TB.
Two years ago, University of Paris graduate student Gaspard Kerner discovered people were at much higher risk of getting severely ill when infected with TB if they inherited two copies of a rare variant of the immune gene TKY2, called P1104A. He realized that by tracing the frequency of that variant in 1013 European genomes from the past 10,000 years, he had a “golden” tool to detect how the immune gene coevolved with TB, says Quintana-Murci, who hired Gaspard as a postdoc at the Pasteur Institute.
The researchers found that the P1104A mutation was ancient—they spotted it in DNA from a farmer who lived 8500 years ago in Anatolia (what is now Turkey) and calculated that the mutation arose at least 30,000 years ago. Anatolian farmers and Yamnaya herders spread this gene variant as they moved into Central Europe. By studying changes in the frequency of the variant over time, the researchers estimated that about 3% of the population carried the gene until about 5000 years ago. By the middle of the Bronze Age, about 3000 years ago, 10% of Europeans had the trait. But since then, its frequency plummeted to 2.9%—the same rate as among today’s Europeans.
The steep plunge coincides with when TB’s modern variant emerged, according to ancient DNA studies. Quintana-Murci and his team ran computer simulations on how population size and migration influenced the gene’s frequency. They propose that TB killed or seriously sickened one-fifth of those with two copies of the variant, few of whom had offspring who survived after the end of the Bronze Age, 2000 years ago. As a result, natural selection acted strongly and quickly to weed out the deadly gene variant to low levels, the researchers report today in The American Journal of Human Genetics.
“Infectious disease are the strongest evolutionary pressure humans have to face,” Quintana-Murci says. Arizona State University, Tempe, molecular anthropologist Anne Stone agrees. Having the two copies of the gene variant, she says, “was not good if TB was in town.”
Stone and other outside researchers say the timing for the selection in humans and the emergence of modern TB fit nicely. “It’s cool and exciting to see two very different lines of data yielding similar results,” says paleogeneticist Kirsten Bos of the Max Planck Institute for Evolutionary Anthropology.
Given new databases like the UK Biobank and additional ancient DNA samples worldwide, this may be “just the beginning” of studies tracing the frequency of variants to understand how our immune systems coevolved with specific pathogens, says Sebastien Gagneux, a microbiologist at the Swiss Tropical and Public Health Institute who was not involved in the study.
But there is an urgent need today to know how widespread the P1104A variant is, Kerner says. It is rare in populations tested in India, Indonesia, China, and parts of Africa where TB is endemic. But about one in 600 British people in the UK Biobank database carries two copies of the variant. They are at high risk of severe illness or death if they are exposed to TB, Kerner says.
Culled from https://www.sciencemag.org/
 Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.
Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.