Europe’s digital “green pass” does not recognise the vaccine donated to many African countries through the COVAX initiative.
The African Union (AU) and Africa Centres for Disease Control and Prevention (Africa CDC) have kicked against this.
- Finally, National Assembly passes Petroleum Industry Bill
- French president offers appointment to Abdul Samad Rabiu, Kano-born billonaire
They urged the EU to consider increasing mandatory access to those vaccines deemed suitable for global rollout through the EU-supported COVAX Facility.
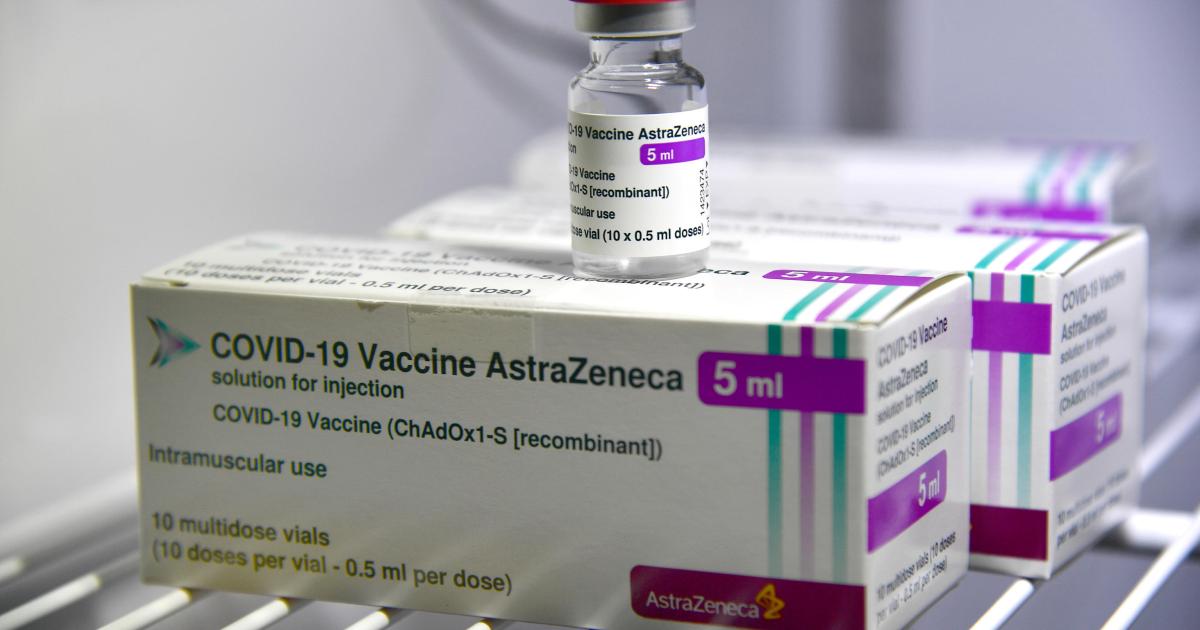
The European Union Digital COVID Certificate enables people who have received two doses of a Covid-19 vaccine approved by its medicines regulator, the European Medicines Agency (EMA), to travel freely within the bloc.
But the pass only recognises AstraZeneca doses (branded Vaxzevria) made by EMA-approved manufacturers in Europe, US, South Korea and China — not those manufactured by the world’s largest vaccine manufacturer, the Serum Institute of India (branded Covishield).
Covishield has been supplied to many African countries.
In a statement obtained by CNN, EMA explained that Vaxzevria is only Covid-19 shot from AstraZeneca for which approval was requested — leading to its authorization in the EU.
“In the EU, the vaccine called Covishield does not currently have a marketing authorization.
“Even though it may use an analogous production technology to Vaxzevria, Covishield as such is not currently approved under EU rules,” the EMA said.
“This is because vaccines are biological products. Even tiny differences in the manufacturing conditions can result in differences in the final product, and EU law therefore requires the manufacturing sites and production process to be assessed and approved as part of the authorization process.
“Should we receive a marketing authorization application for Covishield or should any change to the approved manufacturing sites for Vaxzevria be approved, we would communicate about it?”
Meanwhile, the EU in a separate statement on Wednesday told CNN that the certificate was “not a prerequisite for travel into the European Union” and that “Member States could also allow entry for people vaccinated with vaccines having completed the WHO Emergency Use Listing process,” which includes Covishield.
Travelers may also enter the EU by presenting a negative PCR test.
Covishield has been described as the “backbone” of COVAX contributions to low- and middle-income countries.
The AU and Africa CDC urged the EU Commission, “to consider increasing mandatory access to those vaccines deemed suitable for global rollout through the EU-supported COVAX Facility.”
“The current applicability guidelines put at risk the equitable treatment of persons having received their vaccines in countries profiting from the EU-supported COVAX Facility, including the majority of the African Union (AU) Member States,” they said in a joint statement.
CNN quoted an AstraZeneca spokesperson to have said: “We are working closely with the EMA as they develop guidance to support opening of borders and relaxing restrictions, and this includes guidance on inclusion of Covishield as a recognised vaccine for immunisation passports.”
 Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.
Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.


