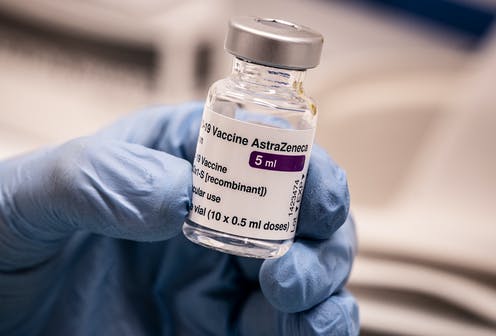
The AstraZeneca coronavirus vaccine is safe and effective and its benefits outweigh the risks, according to Europe’s medicines regulator.
The regulator made its position known days after 12 European countries suspended the vaccine over blood clot concerns.
- WHO reacts to EU suspension of COVID-19 vaccine, says safe to use
- Aisha Buhari Returns To Nigeria After Six Months in Dubai
But on Thursday, the regulator said AstraZeneca vaccine is not linked to an increased risk of blood clots.
“The committee has come to a clear scientific conclusion: this is a safe and effective vaccine,” European Medicines Agency chief Emer Cooke told a press conference after a probe by the body’s safety committee.
“The committee also concluded that the vaccine is not associated with an increase in the overall risk of thromboembolic events or blood clots.”
But Cooke said the agency’s review, launched after about 30 cases of unusual blood clots and low platelet counts in recipients of the vaccine prompted more than a dozen EU countries to suspend its use, had uncovered “a small number of cases of rare and unusual but very serious clotting disorders”.
She said the EMA still could “not rule out definitively a link between these cases and the vaccine”, and was therefore recommending “to raise awareness” of possible risks. A new warning in the vaccine information would draw attention to “possible rare conditions” to help patients and healthcare professionals prevent and mitigate any possible side effects.”
Austria, the Baltic states, Denmark, France, Germany, Ireland, Italy, the Netherlands, Portugal, Spain and Sweden, along with non EU-member Norway, are among the European countries to have either paused the vaccine or banned specific batches.
“This pandemic is costing lives. We have vaccines that can prevent death and hospitalisation – we need to use them,” Cooke said. “A lot of member states are waiting for the outcome of this safety review. Countries can now make an informed decision so as to the safety of the vaccine.”
 Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.
Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.