The Centres for Disease Control and Prevention (CDC) has put out its first vaccine guidance related to a recent outbreak of monkeypox cases across the US and Europe with health workers and others responding to the uptick in cases first in line to get the shots.
The Advisory Committee on Immunization Practices (ACIP), the CDC’s leading experts on vaccines, issued the recommendation Friday.
Others include lab workers who research orthopoxviruses, people who work in lab testing environments, and health care personnel who are treating infected patients.
The JYNNEOS vaccine in question is tailored to both smallpox and monkeypox – just as many other smallpox drugs are also believed to be effective against the rare virus.
It comes as the US records its tenth presumptive case of the virus, with a man in Colorado having a suspected infection after a recent trip to Canada, state officials announced on Thursday night.
The panel notes that orthopoxvirus vaccines, like JYNNEOS, were regularly distributed to children in the US to combat smallpox in the past.
Officials still recommend that some parts of the population do continue to receive the shots, though, including people who may be exposed to these viruses at work.
America has a stockpile of over 1,000 doses of the two-dose vaccine in place for a situation like this.
On Monday, the CDC reported that the country had planned to distribute the shots to the most high-risk group.
The rollout of the vaccines to the high-risk groups is expected to begin soon.
Globally, more than 300 cases have been detected across more than two dozen countries.
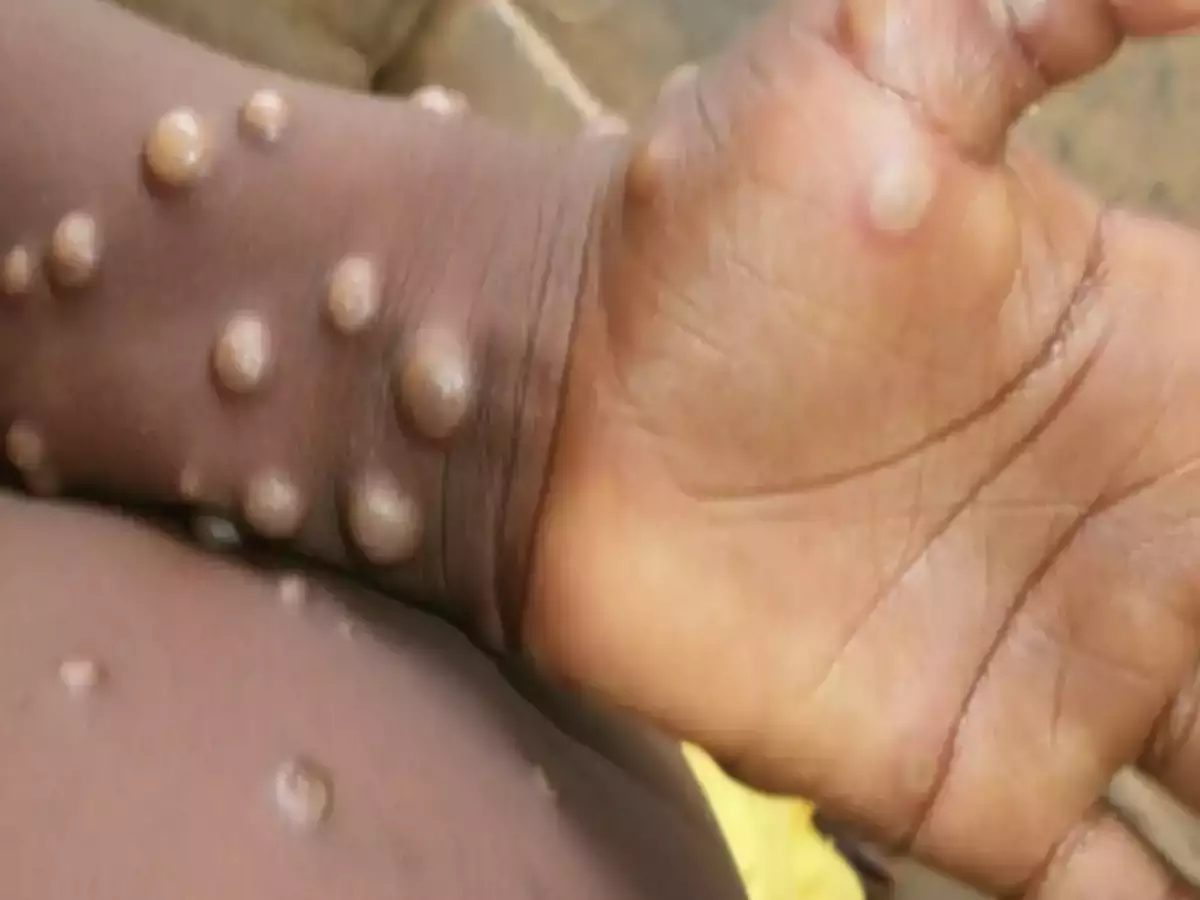
The virus is primarily spread via touch with infected lesions, making it tougher for it to spread than other infectious diseases like COVID-19.
Monkeypox is a rare virus typically only found in West and Central Africa.
No deaths from monkeypox have been reported as part of this recent outbreak across the western world.
 Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.
Join Daily Trust WhatsApp Community For Quick Access To News and Happenings Around You.


